Boron Family
Table of Contents
1. Boron
Boron is mainly extracted from Borax(\( \ce{Na_2B_4O_7 * 10H_2O} \) ) \[ \ce{Na_2B_4O_7 * 10H_2O ->[\Delta] H_3BO_3 ->[\Delta] B_2O_3} \] \[ \ce{Na_2B_4O_7 ->[H^{+}] H_2B_4O_7 ->[\Delta] H_3BO_3} \]
Reduction: This \( \ce{B_2O_3} \)(boric anhydride) on reduction with \( \ce{Mg} \) gives amorphous boron. \[ \ce{B_2O_3 + 3Mg -> 2B\text{(amorphous)} +3 MgO} \]
The reaction involved are \[ \ce{Na_2B_4O_7 + 2HCl -> 2NaCl + H_2B_4O_7\text{(tetraboric acid)} } \] \[ \ce{H_2B_4O_7 + 5H_2O -> 4H_3BO_3 -> B_2O_3 + 3H_2O} \] v High purity crystalline Boron is obtained by following reaction: \[ \ce{2BBr_3_{\text{(g)}} + 3H_2_{\text{(g)}} ->[Ta\text{ or } W \text{ wire} ][1200^{\circ}C] 2B_{\text{(s)}} + 6HBr_{\text{(g)}}}\]
1.1. Compounds of Boron
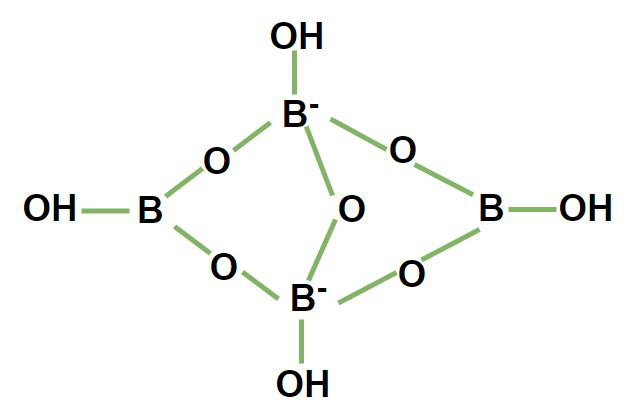
1.1.1. Borax or Tincal or Suhaga (\( \ce{Na_2B_4O_7 * 10H_2O} \) )
- It is a white crystalline solid which contains tetranuclear units \( \ce{[B_4O_5(OH)4]^{2-}} \) and it’s corrected formula is \( \ce{Na_2[B_4O_5(OH)4] * 8H_2O} \)
- Borax dissolves in water to give an alkaline solution. \[ \ce{Na_2B_4O_7 + 7H_2O -> 4H_3BO_3 + 2NaOH} \]
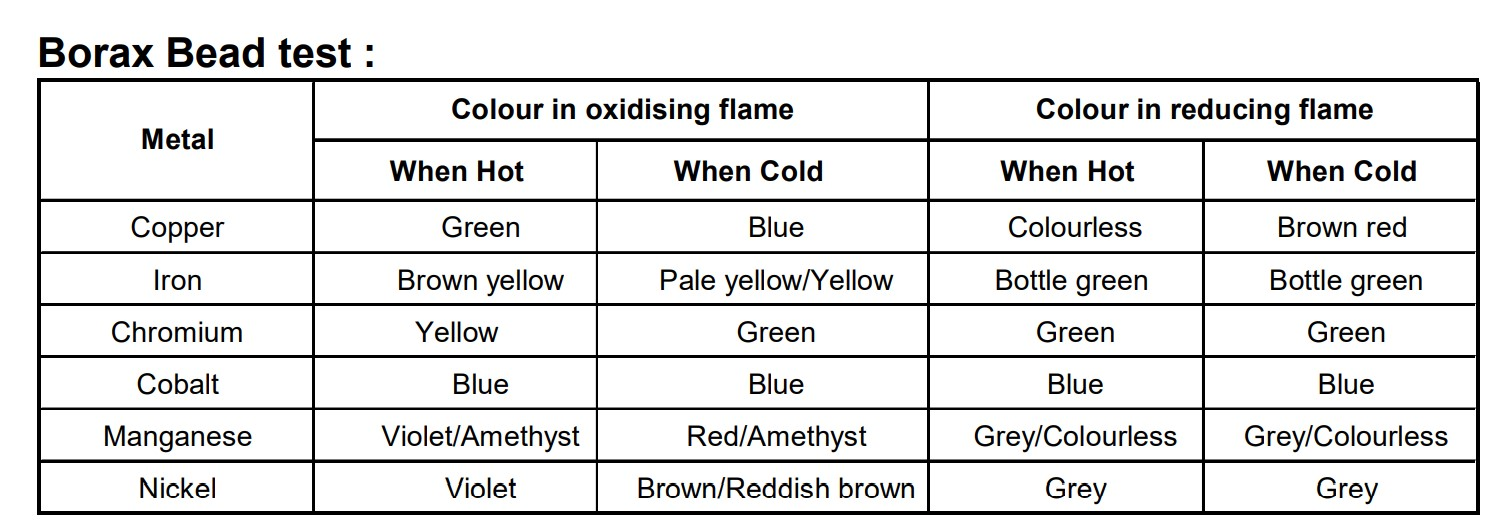
- Borax bead test
When borax is heated, it swells and then it is converted to transparent glassy bead of composition (\( \ce{2NaBO_2 + B_2O_3} \) ). \[ \ce{Na_2B_4O_7 * 10H_2O -> Na_2B_4O_7 + 10H_2O} \] \[ \ce{Na_2B_4O_7 -> $\underbrace{\ce{ 2NaBO_2 + B_2O_3}}_{\text{Glossy bead} } $} \] This glassy bead combines with transition metal oxides to form characteristic coloured beads. \[ \ce{CuO + B_2O_3 -> Cu(BO_2)2 \text{(metaborate blue)} } \] The color of bead is different in oxidizing and reducing flame.
1.1.2. Orthoboric acid (\( \ce{H_3BO_3} \) )
- It is prepared by the acidification of aq. Borax solution. \[ \ce{Na_2B_4O_7 + H_2O -> NaOH + H_3BO_3} \]
- Acidic hydrolysis of borax: \[ \ce{Na_2B_4O_7 + 2HCl + 5H_2O -> 2NaCl + 4H_3BO_3} \]
- It is obtained by hydrolysis of most boron compounds like halides and hydrides.
\[ \ce{BF_3 + H_2O -> H_3BO_3 + HF} \] \[ \ce{B_2H_6 + H_2O -> H_3BO_3} \]
- It is a weak monobasic acid and act as a lewis acid by accepting lone pair from water molecule. \[ \ce{B(OH)3 + 2H_2O -> [B(OH)4]^{-} + H^{+}} \]
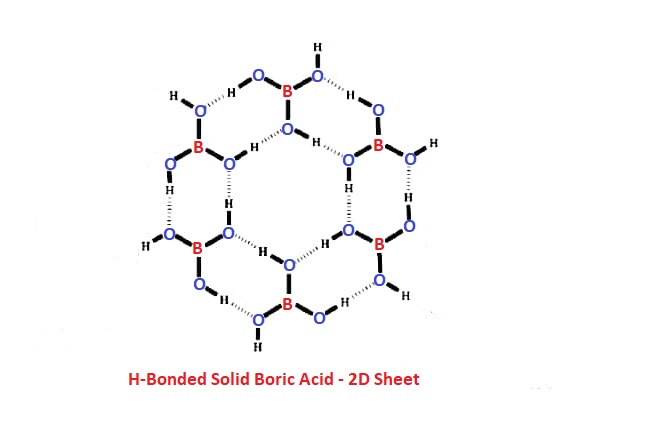
- It has a layered structure in which planar \( \ce{BO_3} \) units are joined by H-bonds.
On heating at \( 100^{\circ}C \), it gives metaboric acid which converts into tetraboric acid on heating at \( 160^{\circ}C \) and on further heating into boric oxide(boric anhyride). \[ \ce{H_3BO_3 ->[\Delta] HBO_2 ->[\Delta] H_2B_4O_7 ->[\Delta] B_2O_3} \]
1.1.3. Boron Hydrides
Boron forms a large number of volatile covalent hydrides known as Boranes. There are two series of Boranes.
| \( \ce{B_nH_{n+4}} \) (more stable) | \( \ce{B_nH_{n+6}} \) (less stable) |
Diborane(\( \ce{B_2H_6} \) ) is the most important.
- Diborane(\( \ce{B_2H_6} \) )
- Prepared by reduction of Boron trichloride. \[ \ce{4BCl_3 + 3LiAlH_4 -> 2B_2H_6 + 3AlCl_3 + 3LiCl} \]
- In laboratory it is prepared by oxidation of \( \ce{NaBH_4} \) by \( \ce{I_2} \) . \[ \ce{2NaBH_4 + I_2 -> B_2H_6 + 2NaI + H_2} \]
- On industrial scale, it is prepared by the reduction of \( \ce{BF_3} \) by \( \ce{NaH} \). \[ \ce{2BF_3 + 6NaH -> B_2H_6 + 6NaF} \]
Properties
- Combustion: Diborance is a colourless toxic gas and spontaneously catches fire on exposure to air. \[ \ce{B_2H_6 + 3O_2 -> B_2O_3 + 3H_2O} \qquad \Delta H = -1976 kJ \text{mol}^{-1} \]
- Hydrolysis: Diborane is easily hydrolyzed and forms boric acid. \[ \ce{B_2H_6_{\text{(g)}} + 6H_2O_{\text{(l)}} -> 2B(OH)3_{\text{(aq)}} + 6H_2_{\text{(g)}}} \]
Reaction with \( \ce{NH_3} \) : First an additional product is formed at lower temperature. \[ \ce{B_2H_6 + 2NH_3 -> B_2H_6 * 2NH_3} \text{ or } \ce{[BH_2 (NH_3)2 ] + [BH_4]^{-}} \] This addition compound on heating at around 450K gives borazole which is known as Inorganic benzene or borazine. \[ \ce{B_2H_6 + 6NH_3 -> 2B_3N_3H_6 + 12H_2} \]
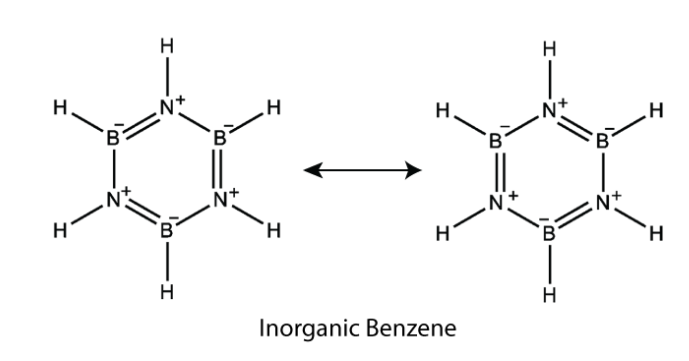
- Reaction with \( \ce{N_2} \) : \[ \ce{B + N_2 -> $\underbrace{\ce{(BN)}}_{\text{Boronitride} }$ ->[N_2] $\underbrace{\ce{(BN)_x}}_{\text{Inorganic Graphite} }$ } \]
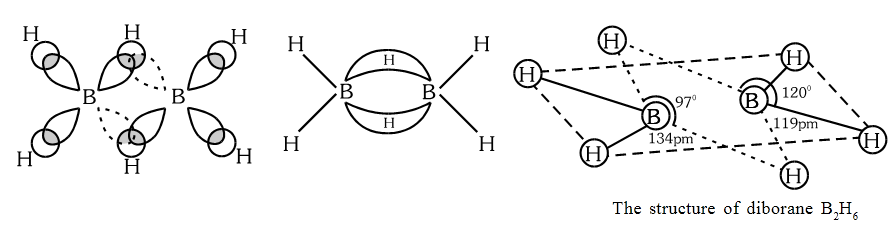
Structure of Diborane
The four terminal hydrogen atoms and the two boron atoms lie in one plane. Above and below this plane, there are two bridging atoms. The four terminal \( \ce{B-H} \) bonds are regular two centre-two electron (2c-2e) bonds (normal covalent bonds) while the two bridge (B-H-B) bonds are different. Each bridge hydrogen is bonded to the two atoms by sharing of only two electrons and therefore is called 2 centre 3 electron (2c-3e) bond.